Retatrutide 10mg is a triple-agonist peptide that activates GLP-1, GIP, and glucagon receptors. This multi-use vial contains 10mg of lyophilized Retatrutide intended for laboratory research.
Research Brief
Retatrutide represents the newest generation of incretin-based compounds. By acting on three receptor pathways at once, it delivers overlapping effects on appetite control, fat metabolism, and energy expenditure.
Early clinical data reported weight loss levels that exceeded 20% of baseline body weight in under one year — higher than most GLP-1 or dual agonist comparators. Researchers are now studying whether Retatrutide’s triple mechanism could establish it as a new benchmark in obesity and metabolic research.
Key Data Points
-
Compound class: Triple receptor agonist (GLP-1, GIP, glucagon)
-
Strength: 10mg per vial
-
Reported weight reduction: 22–24% at 48 weeks (clinical trial data)
-
Unique property: Adds glucagon receptor activation → higher resting energy expenditure
-
Status: Under research investigation, not approved for general medical use
What Makes Retatrutide Different?
-
GLP-1 effect → reduced hunger and improved satiety
-
GIP effect → increased insulin sensitivity and fat metabolism
-
Glucagon effect → higher calorie burn at rest and improved lipid breakdown
The triple combination makes Retatrutide distinct from agents like Semaglutide (GLP-1 only) or Tirzepatide (GLP-1 + GIP).
Handling Guidance
-
Store unreconstituted vials in a cool, dark environment.
-
Reconstitute with bacteriostatic water using sterile technique.
-
Refrigerate reconstituted solution at 2–8°C.
-
Use within the stability period defined by research protocols.
Retatrutide 10mg – Research FAQs
1. Why is Retatrutide described as a “triple agonist”?
Because it activates three receptor systems simultaneously: GLP-1, GIP, and glucagon.
2. How much weight reduction has been observed in studies?
Participants in clinical trials lost over 22% of body weight within 48 weeks, one of the highest recorded outcomes among incretin-based compounds.
3. How does the glucagon receptor pathway change outcomes?
Glucagon receptor activation raises resting energy expenditure, meaning more calories burned even without increased activity.
4. What metabolic markers are being tracked in Retatrutide studies?
Research monitors HbA1c (blood sugar control), triglycerides, LDL cholesterol, and visceral fat distribution.
5. How does Retatrutide compare to Tirzepatide?
Tirzepatide is dual-acting (GLP-1 + GIP). Retatrutide adds glucagon receptor activation, leading to greater weight loss and potentially more robust fat oxidation.
6. Is Retatrutide currently approved for treatment?
No. Retatrutide is investigational and available only in research settings.
7. Why is a 10mg vial format valuable for research?
The higher dose allows multi-week or high-dosage trial protocols without requiring multiple vials.
Related products
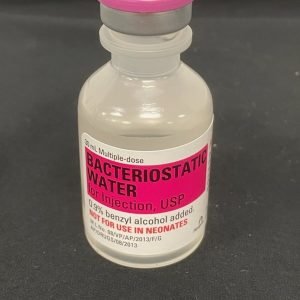
Bacteriostatic Water for Injection 30mL – Sterile, USP
In stock
Key Features & Benefits of Bacteriostatic Water
-
✅ USP-Grade Sterility
Complies with U.S. Pharmacopeia standards for safe use in research and compounding. -
✅ 0.9% Benzyl Alcohol
Acts as a bacteriostatic agent to prevent microbial growth in multi-user applications. -
✅ Multi-Dose Vial
Resealable rubber stopper designed for multiple withdrawals within 28 days. -
✅ Versatile Applications
Perfect for medication reconstitution, injection research, and scientific studies. -
✅ Secure & Discreet Shipping from the United States
Ships fast in sterile, protective packaging with privacy in mind.
BPC-157 + TB-500 Stack
In stock